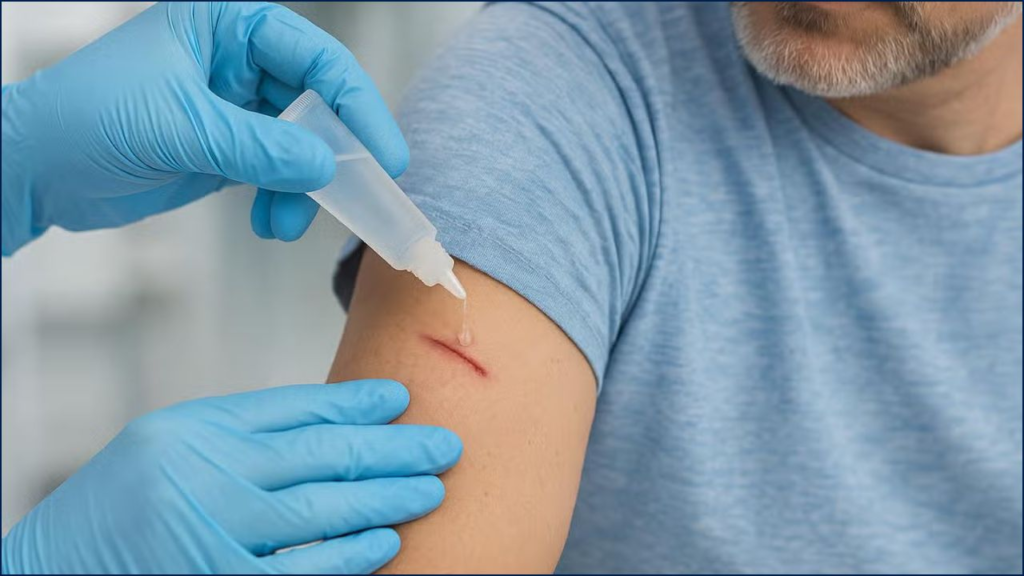
A new wound-sealing bioadhesive developed by international research teams could dramatically reduce the need for traditional stitches, closing wounds in seconds and minimizing scarring. The innovation, unveiled by researchers in the United States, Israel, and Australia, is being hailed as a potential game-changer for trauma care, surgery, and emergency medicine worldwide.
New Breakthrough Seals Wounds
| Key Fact | Detail |
|---|---|
| Seal time | Wounds sealed in under 5 seconds in laboratory tests |
| Primary material | Barnacle-inspired bioadhesive and hydrogel-based polymers |
| Potential use | Emergency trauma care, surgical procedures, battlefield medicine |
| Clinical status | Pre-clinical testing in animal models; human trials expected within 12–18 months |
A Leap Forward in Wound Healing
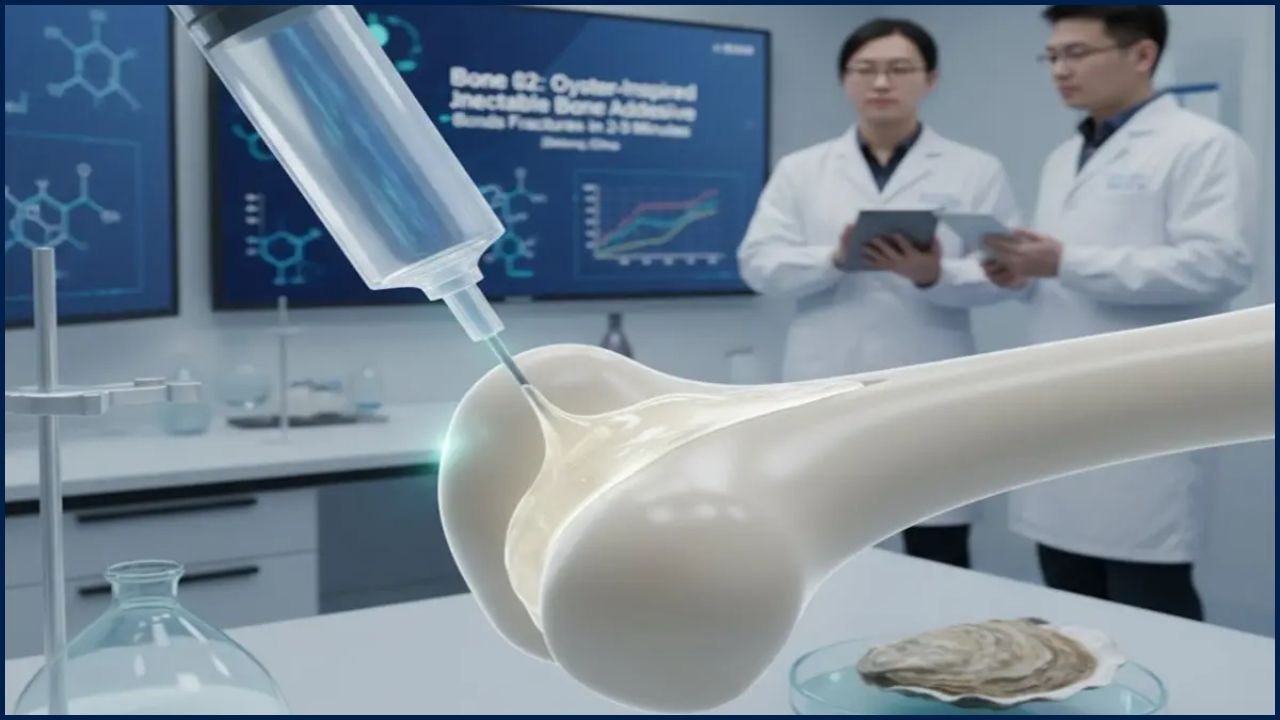
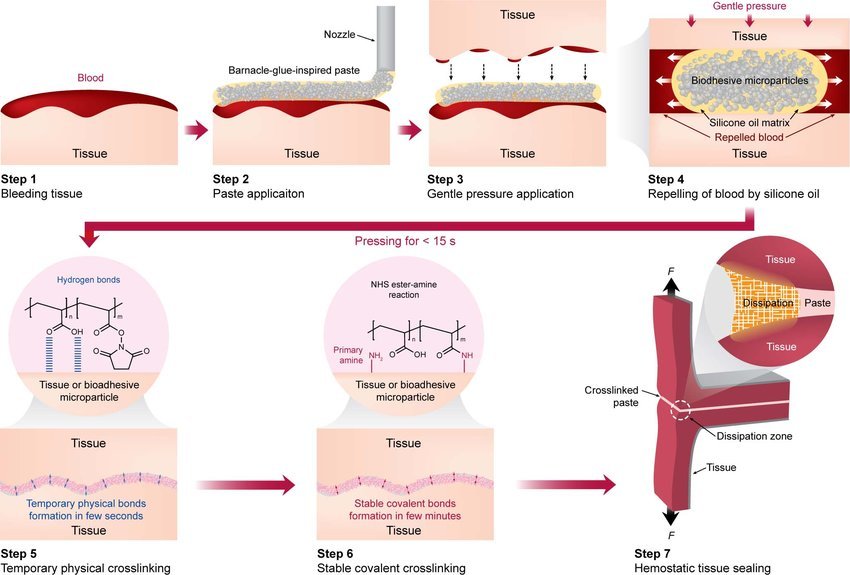
The bioadhesive, inspired by the sticky proteins of barnacles and molluscs, has shown an unprecedented ability to seal bleeding tissue within seconds, even in wet and dynamic environments where most glues fail. Traditional stitches, staples, or fibrin sealants require dry conditions and time—luxuries often unavailable in trauma care.
Researchers at the Massachusetts Institute of Technology (MIT) designed their prototype to mimic the natural adhesion strategies of sea creatures. The glue, made of a polymer matrix suspended in biodegradable microparticles, rapidly forms a watertight barrier once it contacts tissue.
“This material works where sutures simply can’t,” said Dr. Christoph Nabzdyk, a cardiac anesthesiologist and co-lead author of the MIT study. “It’s effective in wet conditions, conforms to irregular wounds, and prevents further blood loss almost instantly.”
How It Works: The Science Behind the Seal
The adhesive consists of biocompatible hydrogels composed of natural polymers such as gelatin and chitosan, mixed with synthetic polymers like polyethylene glycol to achieve elasticity. When applied, the material displaces moisture and cross-links within seconds, creating a durable seal that can withstand blood pressure and tissue movement.
“The real innovation lies in its dual mechanism,” explained Professor Shady Farah of the Technion – Israel Institute of Technology. “First, it repels water through a hydrophobic layer, and second, it bonds tightly through electrostatic and covalent interactions with tissue proteins.”
The sealant remains flexible, biodegrades over time, and can be safely absorbed by the body without removal — a key advantage over stitches or metallic staples.
From Ancient Sutures to Modern Surgery
The quest to heal wounds quickly and cleanly is as old as medicine itself. Ancient Egyptians used flax threads to stitch injuries, while Roman physicians pioneered early surgical needles. In the 20th century, synthetic sutures became the standard, but they still required time, skill, and often left scars.
By the late 1990s, cyanoacrylate-based glues — similar to “super glue” — began limited medical use, but they were brittle, toxic in some formulations, and unsuitable for internal organs. Fibrin sealants, derived from blood plasma, improved safety but degraded too rapidly and worked poorly in heavy bleeding.
The new generation of bioadhesives overcomes many of those limitations, combining strong adhesion, flexibility, and biocompatibility.
| Technology | Typical Use | Key Limitation |
|---|---|---|
| Stitches & Staples | Deep and large wounds | Time-consuming, potential scarring |
| Cyanoacrylate Glue | Skin closure | Toxicity, poor flexibility |
| Fibrin Sealant | Surgical bleeding | Weak in high-pressure conditions |
| Barnacle-Inspired Bioadhesive (2025) | Internal and external wounds | Still under clinical evaluation |
Transforming Trauma and Surgical Care
Emergency Medicine and Field Use
For first responders and military medics, time is critical. “If you can stop bleeding in under 10 seconds, you change the survival curve,” said Dr. Anthony Weiss, biomedical engineer at the University of Sydney, whose team co-developed the “MeTro” elastic surgical glue.
The MeTro formulation, based on a human protein called tropoelastin, activates under UV light and conforms to tissues that expand and contract — like lungs and arteries. “It’s ideal for mobile, high-stress tissues,” Weiss explained.
Field testing in animal models demonstrated that the adhesive could repair lung punctures and arterial injuries within a minute — results that could revolutionize battlefield and disaster medicine.
Inside the Operating Room
Surgeons see additional potential for reducing infection risk and operation time. “Sutures create tiny openings where bacteria can enter,” said Dr. Lina Alvarez, a trauma surgeon at Johns Hopkins University. “A sealant that closes without puncturing tissue could reduce post-surgical infections significantly.”
According to a 2024 review in The Lancet, infections affect up to 11% of surgical patients worldwide, particularly in low-resource settings. A fast-acting adhesive could help prevent complications and shorten recovery time.
Cost, Access, and Ethical Considerations
While the innovation holds promise, experts warn that accessibility could become a challenge. The materials used—especially the protein polymers—are expensive to synthesize at scale.
“The cost factor will determine whether this becomes a global innovation or a niche product for advanced hospitals,” said Dr. Jaya Menon, a health economist at the World Health Organization (WHO). She emphasized the need for public-private partnerships to make such technologies affordable for developing nations.
Another consideration is training. Emergency personnel must learn to apply the adhesive safely and accurately, as misuse could trap contaminants or seal in infection.
Environmental and Safety Aspects
The adhesive’s environmental profile is also under review. Early tests show that the materials are biodegradable and non-toxic, breaking down naturally after tissue repair. Unlike some synthetic glues, they do not release harmful residues or microplastics into the bloodstream.
Regulators, including the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), will require extensive long-term safety data before granting approval.
“Every new biomaterial must be proven safe not just for patients but for medical waste systems too,” said Dr. Amina Rahman, an environmental toxicologist at the University of Toronto.
Regulatory Pathway and Human Trials
According to MIT, pre-clinical results in animal models have been highly encouraging. The next step involves Phase I human safety trials, expected to begin within 12 to 18 months. If approved, widespread clinical adoption could occur by the early 2030s.
The Technion team has already partnered with Israel’s Ministry of Health to develop standardized testing protocols. Meanwhile, several U.S. biotech firms are in talks to commercialize the technology under joint licensing agreements.
“The transition from research to real-world use requires rigorous validation,” said Professor Farah. “But if successful, it could redefine surgical practice for decades.”
A Global Market Ready for Disruption
The global wound closure market is estimated at 15.5 billion dollars (2023) and projected to reach 22 billion dollars by 2030, according to Grand View Research. Bioadhesives represent the fastest-growing segment, driven by rising surgical volumes and demand for minimally invasive solutions.
Healthcare analysts predict that once approved, the sealant could reduce hospital stays, lower surgical costs, and make emergency care more efficient—especially in low- and middle-income countries.
Governments are also taking notice. The U.S. Department of Defense and European Space Agency (ESA) have reportedly expressed interest in using such adhesives in combat medicine and space missions, where traditional suturing is impractical.
Looking Ahead
While the bioadhesive revolution is still in its early stages, the momentum is undeniable. Within a decade, wound care could move from needle and thread to single-application gels that heal faster and leave fewer scars.
“Medicine is shifting from mechanical repair to biological integration,” said Dr. Weiss. “This is not just about faster healing — it’s about smarter healing.”
If trials confirm safety and effectiveness, the phrase “stitch it up” may soon become a thing of the past.
FAQ About New Breakthrough Seals Wounds
Will this adhesive completely replace stitches?
Not immediately. Experts say deep or contaminated wounds will still require sutures until the technology is refined.
When could it reach hospitals?
Human clinical trials are expected to start in 2026. Full regulatory approval could follow later in the decade.
Is it safe?
Early studies suggest it is biocompatible and biodegradable, but regulators will require long-term safety data.
How much will it cost?
Costs are still unknown. Analysts predict initial prices will be high, but mass production could reduce expenses over time.